9005 For Overseas Travelers (Quasi-drugs/ Cosmetics, etc. )
Please pay due attention to the risks involved and importance in importing pharmaceuticals, medical devices, etc.
-
Effectiveness and safety have not been confirmed in Japan.
-
There is a possibility of purchasing products that are inferior and different from the officially marketed products or even counterfeit products.
-
Use of such products by private individuals without expert advice may pose risks.
You are responsible for your own health.
There are individuals who import pharmaceuticals, cosmetic products, etc. from overseas:
- via the Internet
- by purchasing the products overseas and bringing them into Japan.
However, such products purchased overseas pose the following risks.
- Quality, potency and safety of such products have not been confirmed under Japan's Act on Securing Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics Law.
- There are products that make false claims or exaggerate their potency and safety.
- There are products that have been manufactured with unsanitary methods and under unsanitary conditions.
- The product may be actually counterfeit, different from the official product.
- There are instances in which information is not provided in case of side effects or health problems.
For further information,
[When planning to purchase pharmaceuticals, etc. from overseas]
https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iyakuhin/kojinyunyu/index.html(in Japanese)
The following health hazards have been reported in the past.
- Tablets and capsules claimed to be part of the so-called "Hospital Diet" were found to pose serious health hazards, even resulting in death in some cases.
- Pharmaceutical agents were found in products sold as health food or nutritional supplements, causing health hazards.
Sold as weight control food product
Sold as food that boosts potency
- There had been health hazards from pharmaceuticals requiring medical examination and prescription used without expert advice.
RU486 (Internally taken abortion drug)
Diabetes drug
Detailed information can be found at the following website.
[Health hazard & unauthorized drug information]
http://www.mhlw.go.jp/kinkyu/diet.html(in Japanese)
Pharmaceutical products that are marketed through official channels in Japan have a public scheme for salvage in case of serious health hazards resulting from appropriate drug use (program covering damages from drug side effects).
However, hazards resulting from drugs imported privately are not covered by the program.
Before purchasing drugs from overseas, consult a medical doctor, pharmacologist or other expert for advice.
Private import of pharmaceutical products
Regulations and restrictions under the Act on Securing Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics Law and Customs Law apply on import of pharmaceutical drugs, etc. in order to prevent illegal distribution in Japan, and also to prevent hazards on the health and hygiene of the Japanese people.
Import by a private individual (private import) is allowed strictly for personal use. Drugs, etc. imported privately cannot be sold or given to other persons.
As a general rule, private import requires that necessary documents be submitted to the local Bureau of Health and Welfare (regional branch of the Ministry of Health, Labor and Welfare) in order to receive certification that the import does not violate the Pharmaceutical Affairs Law. However, such imported goods may be cleared at customs as exceptional cases, only with "inspection restricted to customs office," if the following applies.
-
Pharmaceutical products and quasi-drugs
Import of pharmaceutical products by private individuals is allowed in view of the need of cases in which pharmaceutical treatment received overseas must continue, and of cases in which travelers from overseas need to carry regularly used drugs.
Although there are products that are classified as quasi-drugs, such as hair-growth agents, bathing agents and tonic drinks, under Japan's Act on Securing Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics Law, they are perceived identical to pharmaceutical products in private import.
It must be noted that pharmaceutical products that are likely to cause serious health hazards when used without expert advice (such as abortion drugs) may not be imported by private individuals, regardless of quantity, without proven prescription by a medical practitioner.
In addition, no product containing the designated pharmaceutical ingredients , which is sold in foreign countries/regions with advertisement on enhancing cerebral function and other mental effects, shall be imported, unless use of such a product is complying with doctor’s prescription or instruction. However, it would be permissible for foreign travelers to bring such a medicinal product into Japan by his/her self, in the purpose of self-medication during his/her stay
- External preparation drugs(excluding toxic, deleterious drugs, prescription drugs*, buccals, troches and suppositories):Less than 24 units per item
* Prescription drugs: Pharmaceutical products that require medical prescription for usage
- Pharmaceutical products and quasi-drugs other than external preparation drugs
- Toxic or deleterious drug or prescription drug: Dosage for less than one month
- Other pharmaceutical products and quasi-drugs: Dosage for less than two months
- External preparation drugs(excluding toxic, deleterious drugs, prescription drugs*, buccals, troches and suppositories):Less than 24 units per item
-
Cosmetic products
As in the case of pharmaceutical products, import by private individuals is permitted strictly for personal use as a general rule.
Less than 24 units per item (standard size)
(In the case of lipstick, for example, less than 24 units, regardless of brand or color)
-
Medical devices
As in the case of pharmaceutical products, import by private individuals is permitted strictly for personal use as a general rule. However, medical devices for medical practitioners cannot be imported. ※Medical devices that are used at home may be classified as medical equipment for use by medical practitioners.
- Medical devices for home use (such as electrical massage device): 1 set
- Disposable contact lenses: Quantity for use for less than two months
For information on documents necessary for importing pharmaceutical products, etc. on a personal basis and submitting to the regional bureau of health and welfare, please inquire with the official in charge of supervising pharmaceutical affairs at one of the regional bureaus of health and welfare shown below.
| Kanto-Shinetsu Regional Bureau of Health and Welfare (Items imported within the jurisdictional district of Hakodate/Tokyo/Yokohama Customs) | e-mail:yakkan[at]mhlw.go.jp (NOTICE: Please replace [at] with‘@’ when sending an email.) |
|---|---|
| Kinki Regional Bureau of Health and Welfare (Items imported within the jurisdictional district of Nagoya Customs and to the west) | e-mail: kiyakuji[at]mhlw.go.jp (NOTICE: Please replace [at] with‘@’ when sending an email.) |
(Reference) Regional Bureau of Health and Welfare website
https://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/01.html
Drugs controlled and restricted for import
-
Narcotic, and stimulants’ raw materials
Import of narcotic, and stimulants’ raw materials is prohibited for private individuals, excluding cases of import for medical use by the person, bringing in such a drug under prescription from a medical practitioner.
(Drugs brought in by persons other than the individual in question or delivered by international post are prohibited.)
Import in person of narcotics and stimulants’ raw materials for medical use (such as morphine, fentanyl and Lisdexan Fentanyl) requires the approval of the director of the regional bureau of health and welfare.
For further information, please check the narcotics control division website.
https://www.ncd.mhlw.go.jp/zenkoku.html(in Japanese) -
psychotropic drugs
Individuals are prohibited from importing psychotropic drugs for medical use(such as diazepam and triazolam) unless they carry and import them themselves. However, it is necessary to have a copy of prescription by a medical practitioner and other documents certifying that such drugs are particularly necessary for treatment of the individual's illness in order to bring such drugs exceeding dosage for one month or injection syringe into Japan.
-
Stimulants
It is regulated by the Stimulants Control Act and cannot be brought into Japan even for treatment purposes.
-
Designated chemical substances
Import of isobutyl nitrite (known as "Rush")etc. is prohibited.
-
Others
The following animal-based drugs and the products that contain them are prohibited under the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES).
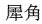
(rhinoceros horn) 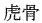
(tiger bone) 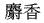
(musk deer secretion) 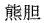
(bear gall bladder), etc.
For consultations on customs procedures, please contact the nearest Customs Counselor.
Please see No. 9301 for inquiries.

