Salt Analysis(Checking the elements in salt)
Salts are indispensable in our lives. The one with think of most often is sodium chloride but they also include calcium chloride and magnesium chloride. As salts are distinguished by their ratio of sodium chloride against other components, it is important to measure the content of sodium chloride.
[ Let's try burning the basic ingredients of salts: ]
Let's burn sodium and potassium (elements), two components of many salts, to see what colors their flames are.

Put some sodium and potassium on platinum wires and burn them.
[ The flame colors are quite different. ]

Sodium

Potassium
Sodium burns with an orange flame and potassium burns with a pink flame. The different colors of the flames means that the wavelengths of light are different. Checking the corresponding wavelengths clarifies the contents (sodium, potassium, etc.).
In addition, the light intensity is proportional to the amount of an element present. In others words, measuring light intensity clarifies the content level of the element.
[ For measuring light wavelength and intensity: ]
An inductively coupled plasma atomic emission spectrometer is used to make this sort of measurement.

Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-AES)
An ICP-AES is used to measure the wavelength and intensity of the flame simultaneously. By using the element-dependent lights (wavelengths), this machine measures the types and amounts of the elements that a substance contains.
Since strong lights are emitted, the machine window has a green filter to protect the eyes of anyone who might look inside. This green filter blocks the true flame color. However, the measured wavelength corresponds to the true color of burning sodium and potassium on platinum wires.
[ Let's see the measurement data. ]
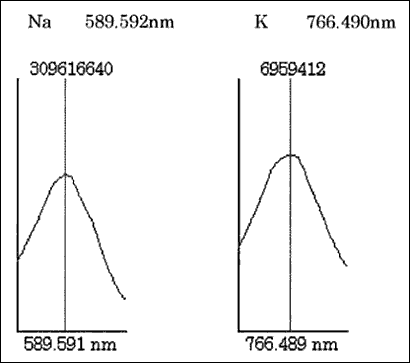
Analytical measurement data of salts
| Results of quantitative analysis | Concentration unit(ppm) | |||
|---|---|---|---|---|
| No. | Sample | Na | K | |
| 1 | Std00 | 10.000 | 10.000 | |
| 2 | Std01 | 0.000 | 0.000 | |
| 3 | Na, K | 9.987 | 10.127 | |
- Electron Microscope (Aluminum oxide or artificial corundum?)
- DNA Analysis (Determining the species of tuna)
- Textile Analysis (Natural or chemical fiber?)
- Comonomer unit wt% Measurement (Copolymer or not?)
- Salt Analysis (Checking the elements in salt)
- Oil Analysis (What are the differences between gasoline, kerosene, and light oil?)
- Rubber Test (Plastic or synthetic rubber?)
![]()
Major Analytical Equipment: Introduction of equipment and devices used for analyses
